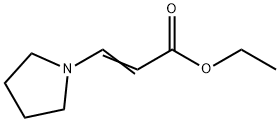
Ethyl trans-3-(1-pyrrolidino)acrylate is used as a precursor to β-vinyllithium derivative; reactions with electrophiles yield cyclopentenones, butenolides, maleic acid derivatives, and oxalacetic acid derivatives.
1. Grinblat, E. I.; Postovski?, I. Y. DOK 1960, 133, 847.
2. Kanner, C. B.; Pandit, U. K. T 1982, 38, 3597.
Ethyl trans-3-(1-pyrrolidino)acrylate is synthesized by reaction of ethyl propiolate with pyrrolidine in acetonitrile at rt yields ethyl β-(1- pyrrolidinyl)acrylate in 55% yield as a colorless solid.1,2
Ethyl trans-3-(1-pyrrolidino)acrylate can be stored under nitrogen protected from moisture.
Ethyl trans-3-(1-pyrrolidino)acrylate distills under reduced pressure.