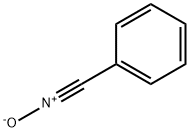
The most widely employed methods of benzonitrile oxide(1) generation involve
triethylamine-mediated dehydrohalogenation of benzhydroxamoyl chloride (or bromide), readily
accessible through chlorination of benzaldoxime, or dehydration of α-nitrotoluene with PhCNO or
PhSO2Cl or ClCO2Et and a catalytic amount of Et3N (Mukaiyama reaction). A reaction of (2) with alkali
metal fluorides also provides a facile quantitative generation of (1). Although (1) has been obtained as a
crystalline solid, it is commonly generated in situ. Et3N almost immediately reacts with (2), and should
be added very slowly to an ice-cooled solution of (2) to maintain a low stationary concentration of (1) and
thereby suppress its dimerization into 3,4-diphenylfuroxan (3). Benzonitrile oxide can also be generated
photochemically by irradiation of (2) and Hexabutyldistannane, or via electrochemical oxidation of
benzaldoxime.