Leritine HCl,Merck Sharp and
Dohme,US,1958
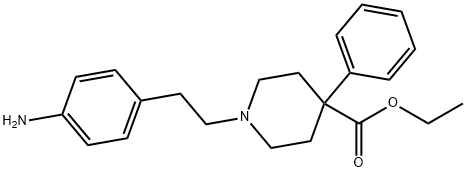
A synthetic analgesic drug. 4-phenylpiperidine derivative that is an analog of Meperidine (M223900) with increased analgesic activity.
ChEBI: A piperidinecarboxylate ester that is the ethyl ester of isonipecotic acid in which the hydrogen alpha- to the carboxyl group is substituted by a phenyl group, and the hydrogen attached to the nitrogen is substituted by a 2-(4-aminophenyl)
thyl group.
A mixture of 7.8 grams (0.05 mol) of β-(p-aminophenyl)ethyl chloride
hydrochloride, 12.5 grams (0.025 mol) of 4-phenyl-4-carboethoxypiperidine
carbonate, 10.5 grams (0.125 mol) sodium bicarbonate, and 100 cc of
anhydrous ethanol are mixed, stirred and heated under reflux for a period of
approximately 40 hours and then concentrated in vacuum to dryness. The
residual material is triturated with 50 cc of water, decanted, washed by
decantation with an additional 50 cc of water, and then dried in vacuum to
give N-[β-(p-aminophenyl)ethyl]-4-phenyl-4-carboethoxypiperidine.
The N-[β-(p-aminophenyl)ethyl]-4-phenyl-4-carboethoxypiperidine is dissolved
in 50 cc of hot anhydrous ethanol, an excess (about 20 cc) of 20% alcoholic
hydrochloric acid solution is added; upon scratching the side of the container
crystals form. One hundred cubic centimeters of ether are then added to the
mixture, the ethereal mixture is cooled, and the crystalline material which
precipitates is recovered by filtration, washed with ether, and dried to give
12.7 grams of N-[β-(p-aminophenyl)ethyl]-4-phenyl-4-carboethoxypiperidine
dihydrochloride which can be further purified by recrystallization from ethanol
or methanal to give substantially pure material; MP 275-277°C.
Poison by ingestion,subcutaneous, intravenous, and intraperitoneal routes.When heated to decomposition it emits toxic fumes ofNOx.