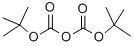
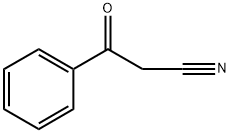
A tetrahydrofuran (100 mL) solution of 3-oxo-3-phenylpropionitrile (7.26 g, 50 mmol) was slowly added dropwise to an ice bath cooled 1.0 M lithium aluminum hydride tetrahydrofuran (100 mL) solution over 15 minutes. The reaction mixture was stirred at room temperature for 15 minutes, then warmed to 60 °C and continued stirring for 2 hours. Upon completion of the reaction, the mixture was cooled in an ice bath and the reaction was quenched sequentially by slow dropwise addition of water (3.8 mL), 4M sodium hydroxide solution (3.8 mL) and water (11.4 mL). After quenching, the mixture was continued to be stirred at room temperature for 20 min, after which the solid precipitate was removed by filtration and the solids were washed with tetrahydrofuran. The filtrate was mixed with di-tert-butyl dicarbonate (Boc2O, 13 g, 59.6 mmol) and stirred at room temperature overnight. The reaction solution was concentrated under reduced pressure and the residue was purified by silica gel column chromatography to afford tert-butyl racemic (3-hydroxy-3-phenylpropyl)carbamate (yield 7.9 g, 62.9%) using hexane-ethyl acetate (70:30) as eluent.