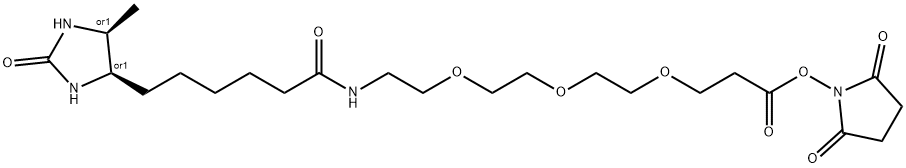
Desthiobiotin is a single-ring, sulfur-free analog of biotin that binds to streptavidin with nearly equal specificity but less affinity than biotin (Kd = 1011 vs. 1015 M, respectively). Consequently, desthiobiotinylated proteins can be eluted readily and specifically from streptavidin affinity resin using mild conditions based on competitive displacement with free biotin. For pull-down experiments with biological samples, this soft-release characteristic of desthiobiotin also helps to minimize co-purification of endogenous biotinylated molecules, which remain bound to streptavidin upon elution of the target protein complexes with free biotin.