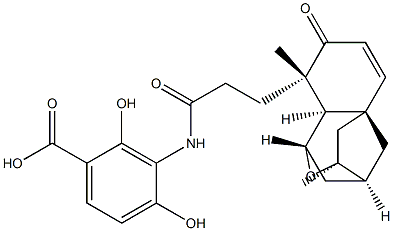
Platensimycin is a novel, broad spectrum, Gram-positive antibiotic produced by strains of Streptomyces platensis. Its discovery was heralded by high profile publication and commentary in the scientific and lay press. Platensimycin was discovered by a target-based, whole-cell screening strategy using an antisense differential sensitivity assay, based on the inhibition of fatty acid synthesis. Platensimycin inhibits bacterial growth by selectively inhibiting the elongation enzyme, b-ketoacyl acyl carrier protein synthase (FabF) of the fatty acid synthesis pathway.
ChEBI: A monocarboxylic acid amide obtained by the formal condensation of the amino group of 3-amino-2,4-dihydroxybenzoic acid with the carboxy group of the oxatetracyclic cage component. It is an antibiotic isolated from Streptomyces platensis a
d exhibits inhibitory activity against fatty acid synthase.
This thiol-reactive Streptomyces platensis-derived antibiotic (FW = 438.50 g/mol; CAS 835876-32-9), also named 3-[[3-[(1R,3R,4R,5aR,9R,9aS)- 1,4,5,8,9,9a-hexahydro-3,9-dimethyl-8-oxo-3H-1,4:3,5a-dimethano-2- benzoxepin-9-yl]-1-oxopropyl]amino]-2,4-dihydroxybenzoic acid, inhibits Staphylococcus aureus b-ketoacyl-[acyl-carrier-protein] synthase II, or FabF (IC50 = 290 nM). This enzyme, which allows bacteria to produce the fatty acids needed for making cell membranes, is thus a uniquely druggable prokaryotic target. Platensimycin has potent, broad-spectrum Gram-positive activity in vitro and exhibits no cross-resistance to other key antibiotic-resistant bacteria including Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate S. aureus, vancomycin-resistant Enterococci, as well as linezolid-resistant and macrolide-resistant pathogens. Platencin is a more potent analogue of Platensimycin. While effective in vivo when continuously administered, its efficacy is reduced when administered by more conventional means. Mechanism of Action: The Cys163 within the FabF active site is activated through the dipole moment of helix N-a-3, lowering its pKa, also increasing its nucleophilicity by the stabilizing effects of FabF’s catalytically required oxyanion hole. Interestingly, the crystal structure complex with platensimycin employed a C163Q mutant which gave a 50-fold increase in apparent binding.