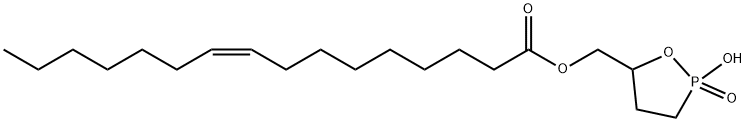
Cyclic phosphatidic acids (cPAs) are naturally occurring analogs of lysophosphatidic acid (LPA) in which the
sn-
2 hydroxy group forms a 5-
membered ring with the
sn-
3 phosphate.
1,2 Carba-
derivatives of cPA (ccPA) are modified at the
sn-
2 (2-
ccPA) or
sn-
3 (3-
ccPA) linkage, preventing the opening of cPA to produce lysophosphatidic acid (LPA).
3 Palmitoleoyl 3-
carbacyclic phosphatidic acid (3-
ccPA 16:1) is a cyclic LPA analog that contains the 16:1 fatty acid, palmitoleate, at the
sn-
1 position of the glycerol backbone.
3 At 25 μM, it inhibits the transcellular migration of MM1 cells across mesothelial cell monolayers in response to fetal bovine serum (86.9%) or LPA (99.9%) without affecting proliferation.
3 3-
ccPA 16:1 significantly inhibits autotaxin (IC
50 = 620 nM),
4 an enzyme that is important in cancer cell survival, growth, migration, invasion and metastasis. When delivered intraperitoneally, 3-
ccPA 16:1 significantly reduces the number of lung metastases formed in mice injected with B16F10 melanoma cells in the tail vein.
4