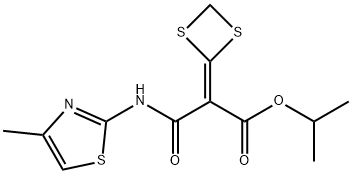
Mivotilate is an orally-active hepatoprotective agent introduced for the treatment of liver cirrhosis and hepatitis-B infection. It is a
dithietanylidene malonate analogue of malotilate and is without the
haemotological adverse effects associated with the latter. Mivotilate can be
obtained by partial saponification of the corresponding diisopropylmalonate,
conversion of the acid function into mixed anhydride then to the amide with an
aminothiazole. It displays potent hepatoprotective activity in rat and mice liver
injury models by a mechanism involving the inactivation of Kupffer cells.
Mivotilate was shown to exert multiple effects on the hepatic cytochrome P450
system, particularly to inhibit CYP2E1 expression and to up-regulate the
CYP1A1 expression. The low oral bioavailability of Mivotilate in rats (F<0.22%)
could be primarilly attributed to poor absorption and considerable hepatic and
gastrointestinal first-pass effects. Mivotilate prevents mutagenesis caused by
agents such as benzo[a]pyrene and reduces skin tumors induced by these
agents.
Mivotilate, is a nontoxic, potent activator of the aryl hydrocarbon receptor (AhR), that can be used as a hepatoprotective agent.
ChEBI: Mivotilate is an aromatic amide, an isopropyl ester and a secondary carboxamide.