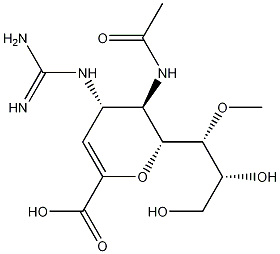
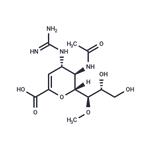
Laninamivir octanoate (CS-8958), an ester prodrug form of the
neuraminidase (NA) inhibitor laninamivir (R-125489), was approved in
Japan in 2010 for treatment of influenza virus infections. Laninamivir
octanoate is given by intranasal administration at a 20 mg or 40 mg dose.
It has a long half-life in humans such that efficacy can be achieved after
only a single dose. In addition to vaccines for immunoprophylaxis, antiviral drugs play an
essential role in the treatment of influenza virus infections. Two
viral proteins have been targeted for therapeutic intervention: the M2
ion channel and NA.
Laninamivir octanoate is prepared starting from a neuraminic acid precursor. The route from 2,3-didehydroneuramic acid entails a multistep sequence to protect the acid and hydroxyl groups at the 4-, 20-, and 30-positions. Methylation of the remaining 10-hydroxyl by treatment with dimethylsulfate and NaH is followed by conversion of the 4-hydroxyl to an amine Cleavage of the 20,30-dihydroxy protecting group, conversion of the 4- NH2 to the guanidine, and acylation of the 30-OH group afford laninamivir octanoate. This three-step sequence can be reordered such that the guanidine is introduced first, followed by deprotection of the 20,30-diOH groups and acylation. An alternative sequence involves a Boc-protected guanidine intermediate, which is converted in a four-step sequence (deprotection of the acid and 20,30-hydroxyl groups, reprotection of the acid as its diphenylmethyl ether, acylation of the 30-OH and deprotection of the guanidine group) to laninamivir octanoate. Laninamivir can also be synthesized from the a-methyl glycoside of N-acetylneuramic acid methyl ester by an analogous route.