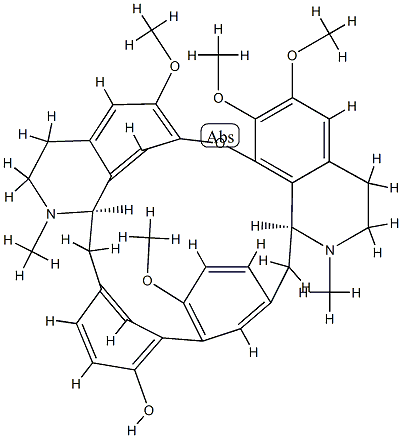
This bisbenzylisoquinoline alkaloid from Ocotea rodiaei was originally considered to have the formulae C36H4006N2 or C36H4406N2. When crystallized from
EtOH it yields cubic crystals but from MeOH it forms slender needles. It has
[α]18D + 134° (c 0.63, CHCI3) or + 157° (c 1.0, CHCI3). The ultraviolet spectrum
in EtOH has absorption maxima at 233 and 285 ITlJ.1 with a shoulder at 292 mJ.1.
The monohydrochloride forms cubic crystals from EtOH, m.p. 255-9°C (dec.);
[α]D + 74° (c 1.0, H20); the dimethiodide, m.p. 321°C (dec.); [α]20D+ 68°
(c 0.147, H20); the dimethochloride, m.p. 286°C (dec.); [α]D + 81.5° (c 0.98,
H20) and the dimethopicrate, existing in two crystal forms, m.p. 180°C and
253°C respectively. Four methoxyl groups and one phenolic hydroxyl group
are present, the alkaloid furnishing the O-methyl derivative, m.p. 172-3°C;
[α]D + 85° (c 0.57, CHCI 3) which also yields crystalline salts, e.g. the hydro�chloride, m.p. 232-6°C (dec.) and the dimethiodide, m.p. 294-8°C (dec.);
[α]D + 47° (H20).
ChEBI: Rodiasine is an isoquinoline alkaloid fundamental parent and a bisbenzylisoquinoline alkaloid.
McKennis et aI., 1. Arner. Chern. Soc., 78,245 (1956)
Grundon, McGarvey.,1. Chern. Soc., 27 39 (1960)
Hearst.,1. Org. Chern., 29,466 (1964)
Structure:
Grundon, McGarvey.,J. Chern. Soc., C, 1082 (1966)
Chan et ai., ibid, 2479 (1967)