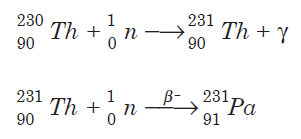Protactinium-233 is produced by the beta decay of the short-lived thorium- 233. Thorium-233 is obtained by neutron capture of natural thorium-232. The nuclear reactions are as follows:
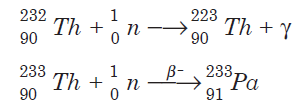
To synthesize Pa-233, thorium nitrate is irradiated with neutron. Pa-233 formed, as shown above, is dissolved in 3M nitric acid. The solution is heated. A manganous salt and permanganate are added to this solution. Manganese dioxide, MnO
2, is precipitated. Pa-233 co-deposits onto this precipitate. The precipitate is washed with water. It is then dissolved in 6M hydrochloric acid.Pa-233 is extracted into diisopropyl ketone. The solvent extract containing Pa-233 is washed with 6M HCl for the removal of trace manganese salts and impurities. From the diisopropyl ketone extract, protactinium-233 is reextracted into an HCl-HF mixture solution containing 6M HCl and 0.1M HF.
Protactinium-231 can be recovered from the residues of uranium refining by various chemical processes. One such recovery process is highlighted below (Maddock, A.G. 1968. Protactinium. In The Encyclopedia of Chemical Elements, ed. C.A. Hampel, pp 580-585. New York: Reinhold Book Corp).
The isotope Pa-231 is extracted with a mixture of 8M HCl and 0.1M HF from the uranium refining residues. Protactinium converts to its fluoride, PaF4. Addition of boric acid or aluminum converts PaF4 into a complex, which is extracted into diisopropyl ketone. The organic solution is washed and the Pa-complex is re-extracted into HCl-HF mixture. After repeated extractions, the diisopropyl ketone solution is treated with oxalic acid to reduce any iron salts present as contaminants. The solution is then treated with potassium hydrogen fluoride, KHF
2 , to precipitate protactinium as K
2PaF
7. The precipitate is filtered and dissolved in sulfuric acid. Treatment with hydrogen peroxide forms a precipitate of protactinium peroxide, thus separating it from niobium. Peroxide on ignition forms diprotactinium pentoxide, Pa
2O
5.
Other reagents can be employed to recover protactinium from uranium refining residues or wastes. For example, treatment with 4M phosphoric or iodic acid precipitates protactinium as phosphate or iodate which is soluble in HF.
Protactinium-231, similar to Pa-233, also can be synthesized by neutron bombardment of thorium-230: