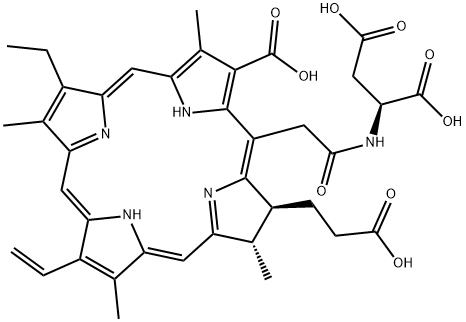
In collaboration with Light Sciences Corp. and Meiji Seika Kaisha, Nippon Petrochemicals
has developed and launched the injectable photosensitizer talaporfin
sodium in Japan for the photodynamic therapy (PDT) of cancer. The initial approval
is for the treatment of early stage lung cancer, but Light Sciences Corp. and
its subsidiaries are also developing talaporfin sodium for other hyperproliferative
diseases, such as, liver metastases arising from colorectal cancer, wet age-related
macular degeneration, and atherosclerosis. Talaporfin sodium is typically supplied
as a lyophilized green powder, and it is synthesized via a carbodiimide-mediated
coupling of chlorin e6 (obtained from precursors that were extracted from natural
sources) with L-aspartic acid. Since chlorin e6 contains three carboxylic acid groups,
the coupling reaction produces a mixture of aspartic acid conjugates. The desired
site of conjugation is the acetic acid side chain of C-20, and the other regioisomers
are removed by chromatographic purification. Compared to other photosensitizers,
talaporfin sodium is associated with minimal cutaneous photosensitivity, is activated
at long wavelengths permitting deeper tissue penetration, and requires a
shorter interval between intravenous administration and photoactivation. It has a
serum half-life of nine hours and is excreted unmetabolized, predominantly by the
biliary system. In the clinical study of patients with early lung cancer, a complete
response was obtained in approximately 86% of the lesions (administration at
40 mg/m2 followed by laser irradiation at 100 J/cm2, 4–6 hours later). In addition to
laser activation, a clinical study involving a variety of refractory solid tumors
demonstrated that intratumoral delivery of light-emitting diodes was effective; a
33% overall response rate was observed with no cutaneous phototoxicity. Regardless
of the mode of activation, the outcome is the same; the activated photosensitizer
reacts with endogenous oxygen to generate singlet oxygen that ultimately
leads to apoptosis and vascular ischemia of the targeted tissue. The most significant adverse effect associated with talaporfin sodium was generalized cutaneous photosensitivity,
and erythema and oedema were common as well. Individual cases of
nausea, vomiting, diarrhea, heartburn, headache, and pruritus were also reported.