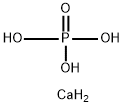
octacalcium phosphate
- Product Nameoctacalcium phosphate
- CAS13767-12-9
- MFCaH5O4P
- MW140.09
- EINECS
- MOL File13767-12-9.mol
Usage And Synthesis
Octacalcium phosphate (OCP) is a layered type of calcium phosphate that shows promise for pharmaceutical and biomedical applications because it offers both excellent biocompatibility and a unique, robust crystal structure that readily accepts substitution by various molecules.
Octacalcium phosphate (OCP) is regarded as a precursor of hydroxyapatite (HA) which is an inorganic constituent of human bones and teeth. OCP is also becoming regarded as one of the important biomaterials. Despite some studies on OCP as biomedical materials, there are few methods for shape forming of OCP. The objective of this study is preparing spherical granules of OCP. The spherical granular shape has an advantage for handling. The spherical granules can achieve easy injection into the defect site by a catheter. In the present study, preparation of spherical granules of OCP from α-tricalcium phosphate (α-TCP) was attempted. The starting material of α-TCP powder was dispersed in the gelatin solution. The resultant slurry was added into vegetable oil, and then the spherical granules of α-TCP/gelatin were formed by the surface tension of the slurry and the shearing force of stirring. By calcining the obtained α-TCP/gelatin granules, the spherical granules with α-TCP single phase were obtained. These spherical granules of α-TCP were immersed in the acetic acid buffer solution whose temperature and pH were controlled. The calcium phosphate spherical granules containing OCP were obtained. The shorter treatment time was favorable for preparing spherical granules containing more OCP.
https://www.worldscientific.com/doi/abs/10.1142/S1793604712600090
https://www.worldscientific.com/doi/abs/10.1142/S1793604712600090
Octacalcium phosphate (OCP), CagH2(P04)6.5H20, is frequently encountered in calcium phosphate systems more basic than dicalcium phosphate dihydrate (DCPD), CaHP04·2H20. It forms and hydrolyzes rapidly under physiological conditions. Octacalcium phosphate (OCP) is proving to be an important intermediary in the formation of tooth and bone mineral and various pathological calcifications.
Octacalcium phosphate was synthesized by precip itation from solutions of calcium acetate (0.04 mol/L) and sodium hydrogen phosphate (0.04 mol/L). The pH value and solution temperature varied in the ranges of 5.0–6.5 and 37–90°С, respectively. Two hours after the beginning of the reaction, the precipitates were collected on a Büchner funnel, washed successively with water and ethanol, and dried at room tempera ture.
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine