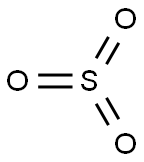
sulfur trioxide
- Product Namesulfur trioxide
- CAS14265-45-3
- MFO3S
- MW80.06
- EINECS
- MOL File14265-45-3.mol
Chemical Properties
| EPA Substance Registry System | Sulfite (14265-45-3) |
Usage And Synthesis
Endogenous sulfite is generated as a consequence of the body’’s
normal processing of sulfur-containing amino acids. In addition,
as discussed below, sulfite can be produced by neutrophils.
Sulfites occur as a consequence of fermentation and also naturally
in a number of foods and beverages. As food additives,
sulfating agents were first used in 1664, and approved for use in
the United States in the 1800s. Sulfite is also noted as a water
treatment additive, for example, to control oxygen levels in
power plant boiler water. Further, sulfur dioxide is acommonair
pollutant produced by numerous processes (burning sulfurbearing
coal, smelting sulfide ores, etc.), and may enter the body
via inhalation. Sulfur dioxide has been reported to react with
water in the ambient air and in the respiratory tract’s mucous
membranes to form sulfite and bisulfite ions.
Inorganic sulfites and bisulfites (such as sodium sulfite,
Na2O3S) are used in photography, the bleaching of wool, and
as preservatives in foods, beverages, and medications. They act
as effective antioxidant compounds and are also used in the
manufacture of pulp for paper and wood products. Their
preservative properties include controlling microbial growth
and the prevention of browning and spoilage.
Under the US Federal Food, Drug, and Cosmetic Act, sulfites are permitted for use as preservatives in food. Like other ingredients, sulfites must be declared in the ingredient statement when added to a food product. In addition, sodium sulfite, ammonium sulfite, sodium bisulfite, potassium bisulfite, ammonium bisulfite, sodium metabisulfite, and potassium metabisulfite are inorganic salts that function as reducing agents in cosmetic formulations. All except sodium metabisulfite also function as hair waving/straightening agents. In addition, sodium sulfite, potassium sulfite, sodium bisulfite, and sodium metabisulfite function as antioxidants in cosmetics. All except ammonium sulfite are widely used in hair care products.
Under the US Federal Food, Drug, and Cosmetic Act, sulfites are permitted for use as preservatives in food. Like other ingredients, sulfites must be declared in the ingredient statement when added to a food product. In addition, sodium sulfite, ammonium sulfite, sodium bisulfite, potassium bisulfite, ammonium bisulfite, sodium metabisulfite, and potassium metabisulfite are inorganic salts that function as reducing agents in cosmetic formulations. All except sodium metabisulfite also function as hair waving/straightening agents. In addition, sodium sulfite, potassium sulfite, sodium bisulfite, and sodium metabisulfite function as antioxidants in cosmetics. All except ammonium sulfite are widely used in hair care products.
Sulfites are generally soluble compounds that interact with the
environment through a variety of processes. The primary function of sulfites is that of a reducing agent, which can
remove dissolved oxygen from waterways. This reduction of
dissolved oxygen (normally produced via normal aeration
through water movement, falls and disturbances, etc.) in turn
generates a favorable environment for anaerobic bacteria, disrupting
the local microbiota. Decreases in dissolved oxygen
caused by the presence of sulfites, typically below 5 ppm dissolved
oxygen, can negatively affect fish and other organisms
present in polluted waterways. Another effect of sulfite
contamination of waterways is the production of hydrogen
sulfide gas, which is a by-product of sulfite-induced redox
processes.
Flue gas desulfurization is an industrial process that produces calcium sulfite (CaSO3) as a by-product. This relatively insoluble sulfite, when deposited in soils, can cause a general shift in local microbiot a; however, calcium sulfite readily undergoes oxidation to calcium sulfate (gypsum), which is generally accepted as a remediation material for poor quality soils. While the resulting gypsum can have beneficial effects on soils for agricultural purposes, large quantities of sulfite species over longer time spans can be expected to directly affect microbial communities in soils, even after conversion to the more benign calcium sulfate. Consequently, the presence of calcium sulfate may produce blooming as a result of oxygen- and sulfurenriched soils and adjoining waterways.
Flue gas desulfurization is an industrial process that produces calcium sulfite (CaSO3) as a by-product. This relatively insoluble sulfite, when deposited in soils, can cause a general shift in local microbiot a; however, calcium sulfite readily undergoes oxidation to calcium sulfate (gypsum), which is generally accepted as a remediation material for poor quality soils. While the resulting gypsum can have beneficial effects on soils for agricultural purposes, large quantities of sulfite species over longer time spans can be expected to directly affect microbial communities in soils, even after conversion to the more benign calcium sulfate. Consequently, the presence of calcium sulfate may produce blooming as a result of oxygen- and sulfurenriched soils and adjoining waterways.
Although the physiological basis for sulfite sensitivity is still
poorly understood, clinical observations have established that
certain medical conditions are associated with a predisposition
to sulfite hypersensitivity. Approximately 500 000 individuals
in the United States (<0.05% of the population) are at higher
risk because they are asthma sufferers who are steroid dependent
or have general airway hypersensitivity. Studies suggest
that sulfur dioxide is the agent that causes the highest physiological
response. It has been shown that bronchoconstriction as
a result of SO2 exposure is controlled by chemosensitive
receptors in the tracheobronchial tree. Sensory C-fiber receptors
and rapidly activating receptors are sensitive to gases such as
SO2, and are found throughout the respiratory tract. Activation
of these receptors triggers central nervous system reflexes that
culminate in bronchoconstriction, mucosal vasodilation,
cough, mucus secretion, apnea, and potential of bradycardia
and changes in blood pressure. Inhaled sulfur dioxide elicited
a stronger reaction in sulfite oxidase–deficient rats than
endogenously accumulated sulfites and S-sulfocysteine (a
reaction product of sulfite with cysteine residues in proteins).
Bisulfites result in three main reactions with biomolecules: sulfonation (sulfitolysis), autooxidation (and generation of free radicals), and cytosine addition. Sulfonation reactions in the body resulting from exogenous bisulfites can be long-lived in vivo. Autooxidation reactions induced by bisulfites may result in lipid peroxidation, and possible damage to plasma membranes as a result. Sulfite addition to cytosine creates uracil, producing a mutation at that site.
Bisulfites result in three main reactions with biomolecules: sulfonation (sulfitolysis), autooxidation (and generation of free radicals), and cytosine addition. Sulfonation reactions in the body resulting from exogenous bisulfites can be long-lived in vivo. Autooxidation reactions induced by bisulfites may result in lipid peroxidation, and possible damage to plasma membranes as a result. Sulfite addition to cytosine creates uracil, producing a mutation at that site.
Preparation Products And Raw materials
Preparation Products
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine