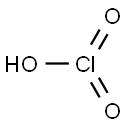
ChEBI: Chlorate is a monovalent inorganic anion obtained by deprotonation of chloric acid. It is a monovalent inorganic anion and a chlorine oxoanion. It is a conjugate base of a chloric acid.
Chlorate, inorganic, n.o.s. is a white crystalline. chloric acid is soluble in water. The material itself is noncombustible, but chloric acid can form a very flammable mixture with combustible materials, and this mixture may be explosive if the combustible material is very finely divided. The mixture can be ignited by friction. Contact with strong sulfuric acid can cause fires or explosions. When mixed with ammonium salts, spontaneous decomposition and ignition may result. Prolonged exposure of the material to heat or fire can result in an explosion.
They are soluble in water.
Metal chlorates are oxidants in the presence of strong acid; liberates explosive chlorine dioxide gas; liberates chlorine dioxide and carbon dioxide by heating a moist metal chlorate and a dibasic organic acid; mixtures of perchlorates with sulfur or phosphorus are explosives [Bretherick 1979 p. 100]; mixtures of the chlorate with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive [Bretherick 1979 p. 806]. A combination of finely divided aluminum with finely divided bromates (also chlorates and iodates) of barium, calcium, magnesium, potassium, sodium, or zinc can explode by heat, percussion, or friction [Mellor 2:310 1946-47].
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.