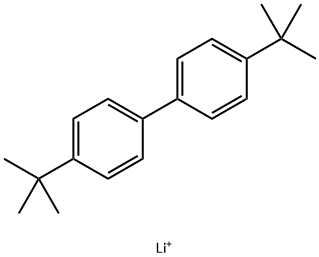
Lithium p,p'-di-tert-butylbiphenyl can be used for the reduction lithiation of thiophene ethers, carbonyls, and phosphonates; the formation of alkyl lithiums, α-lithium ethers, allyl lithiums, 17,18 β-, γ-, and δ-lithium alkoxides; and the cleavage of propeller alkane bonds.
Anhydrous THF and 4,4′-di-t-butylbiphenyl (DBB) are cooled to 0 °C under a blanket of argon and stirred with a glass-coated stirring bar; Lithium foil (or ribbon) is added in portions and the mixture is stirred for 5 h at 0 °C; the appearance of the deep blue-green radical anion appears within 5 min.