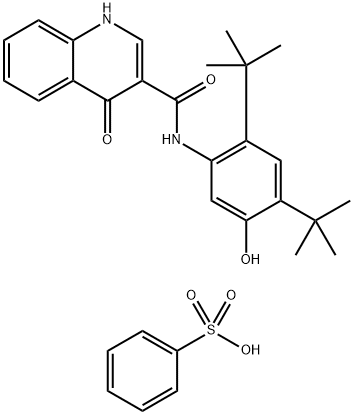
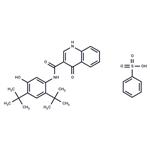
Ivacaftor Benzenesulfonate is a salt of Ivacaftor, a novel agent for the treatment of cystic fibrosis.
ivacaftor (vx-770) is a cftr potentiator approved for patients with the g551d mutation of cystic fibrosis, which accounts for 4-5% cases of cystic fibrosis.
in recombinant cells vx-770 increased cftr channel open probability (p(o)) in both the f508del processing mutation and the g551d gating mutation. vx-770 also increased cl(-) secretion in cultured human cf bronchial epithelia (hbe) carrying the g551d gating mutation on one allele and the f508del processing mutation on the other allele by approximately 10-fold, to approximately 50% of that observed in hbe isolated from individuals without cf [1].
at day 28, in the group of subjects who received 150 mg of vx-770, the median change in the nasal potential difference (in response to the administration of a chloride-free isoproterenol solution) from baseline was -3.5 mv (range, -8.3 to 0.5; p=0.02 for the within-subject comparison, p=0.13 vs. placebo), and the median change in the level of sweat chloride was -59.5 mmol per liter (range, -66.0 to -19.0; p=0.008 within-subject, p=0.02 vs. placebo) [2].
25 nm (f508del-cftr);100 nm (g551d-cftr) [1]