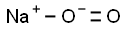Sodium superoxide, NaO2, is a yellow solid.
A solid. Yellowish at room temperatures but may become white when cooled. Prolonged exposure to fire or heat may cause vigorous decomposition and the rupturing of the containers.
Reacts with moisture and carbon dioxide in the air. Reacts vigorously with water to give oxygen and sodium hydroxide.
sodium superoxide violently decomposes above 250°C, evolving oxygen [Mellor 2 Supp. 2:639 1961]. An oxidizing agent. Reacts with carbon dioxide to give sodium carbonate and oxygen. Mixtures with combustible materials (most organic compounds) are readily ignited by friction, heat, or contact with moisture.
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire may produce irritating and/or toxic gases. Toxic fumes or dust may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Runoff from fire control or dilution water may cause pollution.
May explode from friction, heat or contamination. These substances will accelerate burning when involved in a fire. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react explosively with hydrocarbons (fuels). Containers may explode when heated. Runoff may create fire or explosion hazard.
An oxidizer. When heated to decomposition it emits toxic vapors of Na2O.