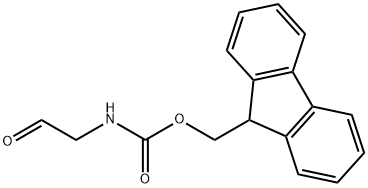
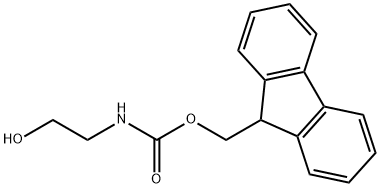
General procedure for the synthesis of N-fluorenylmethoxycarbonylglyceraldehyde from 2-(N-fluorenylmethoxycarbonylamino)ethanol: Diisopropylethylamine (12.4 mL, 70.6 mmol) was added to a dichloromethane (50 mL) suspension of 2-(N-fluorenylmethoxycarbonylamino)ethanol (5.0 g, 18 mmol). The reaction mixture was cooled to -40 °C and a solution of sulfur trioxide pyridine complex (11.2 g, 70.6 mmol) in dimethyl sulfoxide (49.5 mL) was added dropwise over 15 min. After continuous stirring at -40 °C for 1 h, the reaction was quenched with a mixture of ice water and saturated saline. The organic solvent was removed by concentration under reduced pressure, and the resulting off-white precipitate was collected by filtration and dried under vacuum. Finally, purification was carried out by silica gel fast column chromatography to afford N-fluorenylmethoxycarbonylglyceraldehyde (4.7 g, 94% yield) using ethyl acetate/hexane (1:1, v/v) as eluent.
[1] Organic Letters, 2002, vol. 4, # 17, p. 3001 - 3003
[2] Synthetic Communications, 2007, vol. 37, # 20, p. 3493 - 3499
[3] Patent: WO2018/149419, 2018, A1. Location in patent: Paragraph 001283; 001284
[4] European Journal of Organic Chemistry, 2008, # 34, p. 5786 - 5797
[5] Patent: WO2006/66183, 2006, A2. Location in patent: Page/Page column 63