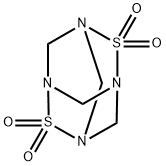
Tetramethylenedisulfotetramine (2,6-dithia-1,3,5,7-tetraazadamantane,
2,2,6,6-tetraoxide, TETS) is a highly toxic heteroadamantane
rodenticide. It is an odorless, tasteless, white
crystalline powder that is slightly soluble in water
(0.25 mg ml-1), dimethyl sulfoxide and acetone. It was originally
synthesized in 1933 as a resinous condensation product
of sulfamide and formaldehyde and used commercially in
pillows and upholstery as an impregnating stiffening and antimold
agent. However, in 1950, a massive poisoning of
German workers in the furniture manufacturing industry was
linked to ‘Crinex’ wool, which contained TETS as a byproduct
of processing. Early experimental studies in rodents revealed
that TETS was an extremely toxic convulsant agent. It was also
discovered at this time that TETS is a highly effective rodent
repellent, which resulted in its use during reforestation
projects to prevent seed predation by rodents. However,
because of its high toxicity in mammals, including humans,
and its persistence in the environment, many countries banned
its production and use in 1984. This ban became worldwide
when China issued similar restrictions in 1991. However,
due to its relative ease of synthesis and low cost, TETS remains
available on the black market, particularly in many rural areas
of China and in regions outside of China that have large Asian
populations.
Despite the worldwide ban on its production and use, TETS
continues to be used illicitly as a rodenticide in various regions
of the world. In China, TETS is known as ‘Dushuqiang’,
‘Meishuming,’ or ‘Shanbudao.’ In 2000, the National Poison
Control Center of China revealed that 74% of commercial
rodenticides contained illegal chemicals, with TETS found in nearly 50% of these pesticides. From 1977 to 2002, it was
estimated that there were thousands of cases of TETS poisoning
in China, resulting in hundreds of deaths. A more recent
analysis indicates that between 1991 and 2010, there were over
14 000 cases of TETS intoxication in China, of which 932
resulted in death. In 2003, the first case of TETS intoxication in
the United States was reported: a healthy 15-month-old girl
was poisoned following accidental ingestion of a rodenticide
imported from China that contained TETS. While many cases
are thought to be due to accidental poisonings, there have been
numerous reports of TETS being used to intentionally poison
humans.
Although TETS has a relatively low solubility in water
(0.25 mg kg-1), it is quite stable, thus making it relatively
persistent in the environment. It is reported that TETS retains
biological activity in water for 6 weeks to 5 months after preparation.
It is believed that TETS bioaccumulates (despite a predicted
octanol:water coefficient of 0.07) and that contact with
poisoned animals can result in intoxication, as demonstrated by
reports of dogs dying after eating TETS-poisoned rats and by
Chinese newspapers warning against consuming meat from
dogs that were suspected to have eaten TETS-poisoned rats.
TETS is a potent convulsant neurotoxicant. TETS has no major
effects on peripheral neuromuscular or autonomic transmission
and its toxicity appears to be due primarily to actions
on the central nervous system. Postmortem studies of TETSpoisoned
patients revealed significant pathology in the brain,
including degeneration in the basal ganglia, subarachnoid
hemorrhages, cerebral and cerebellar bleeding, and brainstem
hemorrhages. In cases of extreme intoxication, edema,
congestion, and hemorrhages are commonly found in not only
the brain but also the heart, lungs, liver, and kidneys. Markers
of severe tissue damage (aspartate aminotransferase, alanine
aminotransferase, creatine phosphokinase, lactate dehydrogenase,
a-hydroxybutyrate dehydrogenase, and creatine phosphokinase
isoenzyme MB) are also higher in patients who
succumbed to TETS poisoning compared to patients who
survived. Additional consequences of TETS intoxication
include low potassium levels (hypokalemia), low phosphorus
levels (hypophosphatemia), abnormal sodium levels (hypo- or
hypernatremia), metabolic acidosis, circulatory hypoxia, and
renal tubular damage as demonstrated by elevated levels of Nacetyl-
b-D-glucosaminidase and retinol-binding protein.
Collectively, these findings are consistent with reports that
death following acute TETS intoxication is primarily due to
multiple organ dysfunction syndrome.
It is generally believed that the convulsant action of TETS is
mediated by noncompetitive reversible antagonism of the
GABAA receptor chloride channel. TETS blocks g-aminobutyric
acid (GABA)-dependent chloride influx in diverse
experimental preparations and inhibits the binding of [35S]
TBPS to GABAA receptors. GABAA receptors are composed of
different subunits (α1–α6, β1–β4, γ1–γ3, δ, ξ, π, and ρ1–ρ3),
and require at least one α, β, and γ subunit to be fully functional.
TETS is active on native GABAA receptors and
recombinantα1β3γ2 receptors, but has no effect on
recombinant receptors composed entirely of β3 subunits.
Picrotoxin, which similarly induces convulsions via GABA
receptor antagonism, is much more selective for the β3
receptor compared to native receptors and the recombinant
α1β3γ2 hetero-oligomer.