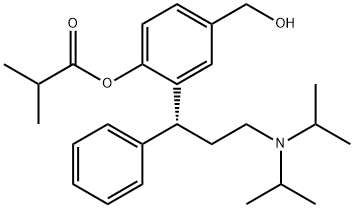
Fesoterodine, launched for the treatment of OAB, is an orally
active pro-drug that is converted in vivo to its active metabolite 5-HMT
through hydrolysis by non-specific esterases. 5-HMT is also an active
metabolite of tolterodine (Detrol), which has been marketed for the
treatment of OAB since 1998. 5-HMT is a potent muscarinic antagonist,
with essentially equivalent affinity for M1, M2, M3, M4, and M5
receptors (Ki=0.32, 0.63, 1.26, 2, and 0.63 nM, respectively). The binding
of 5-HMT is stereoselective; the corresponding S-enantiomer has at least
100 times lower binding affinity for all five receptors. Fesoterodine is
supplied as its fumarate salt in an extended release tablet form. The
recommended starting dose is 4 mg once daily. On the basis of individual
response and tolerability, the dose may be increased to 8 mg once
daily. Following oral administration, fesoterodine is not detected in the
peripheral blood, thereby indicating a rapid and complete bioconversion
to 5-HMT. Bioavailability of 5-HMT is about 52%. After single- or
multiple-dose oral administration of fesoterodine in doses from 4 to
28 mg, plasma concentrations of 5-HMT are proportional to the dose.
Maximum plasma levels are reached after approximately 5 h. No
accumulation occurs after multiple-dose administration. 5-HMT is further metabolized in the liver through oxidation of the hydroxymethyl
group and oxidative cleavage of N-alkyl groups mediated by CYP2D6
and CYP3A4. 5-HMT and its metabolites are primarily eliminated through renal excretion.
The most common adverse events associated with fesoterodine include dry mouth, constipation, and dyspepsia. Fesoterodine is contraindicated in patients with urinary retention, gastric retention, or uncontrolled narrow-angle glaucoma.
The chemical synthesis of fesoterodine starts with the cyclization of 4-(hydroxymethyl)phenol with cinnamaldehyde by means of piperazine in refluxing toluene to produce 2-hydroxy-6-(hydroxymethyl)-4-phenyl-3,4-dihydro-2H-1- benzopyran, which is subjected to reductive amination with To Market, To Market 2008 605 diisopropylamine by means of hydrogen over palladium hydroxide catalyst. The resultant racemic amine intermediate is optically resolved with (R)-(2)-acetoxy-2-phenylacetic acid to yield the corresponding (R)- enantiomer, which is finally acylated with isobutyryl chloride to afford fesoterodine.