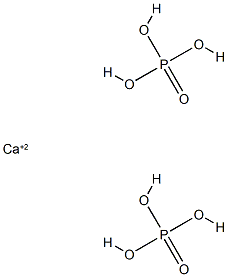
Monocalcium phosphate is one of the components (the
other being calcium sulphate) of single superphosphate.
Single superphosphate is a principal phosphate fertilizer.
It also supplies two secondary elements, namely sulphur
and calcium as it contains 21 % calcium.
Monocalcium phosphate of single superphosphate
dissolves in soil moisture and in that form the roots absorb phosphoric acid. The rest of the solution of
monocalcium phosphate precipitates in soil pores and
forms different phosphate compounds which are not
water-soluble and do not leach out.
Normal superphosphate is made by treating ground rock
phosphate with sulphuric acid.
The above reaction yields monocalcium phosphate
and gypsum.
For the manufacture of normal superphosphates, a
continuous process rather than a batch process is in
vogue. Some of these processes are named after
their inventors, for example, the Tennessee Valley
Authority process, the Maxitz-Standard Den process,
the Broadfield Den process and the Kuhlman Den
process.
The treated mass is sent to a curing shed and stored for
3 to 4 weeks for curing and final drying. The dried
product is excavated, milled, screened, and either bagged
for marketing or sent for granulation.
Granulation is done in a granulator in the presence of
steam and the finished product is then bagged. It contains
16 to 21% phosphorus pentoxide (P2O5) in a watersoluble
form. In addition, it contains 11 to 12% sulphur
as calcium sulphate.