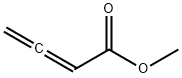
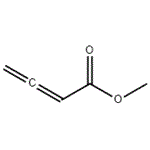
Methyl 2,3-Butadienoate is used as an reactive electrophilic reagents for nucleophilic addition, Michael addition, [2 + 2], [2 + 3], and
[2 + 4] cycloadditions, and hetero-Cope rearrangements.
Methyl 2,3-Butadienoate is most conveniently obtained via a Wittig reaction of [E(R)CHPPh3]+X- or
E(R)C=PPh3 and RR′CHCOCl (R, R′ = H, alkyl) in the presence of Triethylamine in CH2Cl2.[1]Alternatively, the Wittig reaction can be performed with the corresponding ketenes.[2]
Methyl 2,3-Butadienoate can undergo polymerization and so should be stored in the presence
of hydroquinone. For best results, it should be freshly distilled. Use in a fume hood.
Purification of Methyl 2,3-Butadienoate: rapid distillation under reduced pressure in a short-path distillation apparatus or column
chromatography on Alox N with hexane/Et2O (9:1). Hydroquinone may be added for distillation.
1. (a) Lang, R. W.; Hansen, H.-J. HCA 1980, 63, 438. (b) Lang, R. W.; Hansen, H.-J. OS 1984, 62, 202
2. Hamlet, Z.; Barker, W. D. S 1970, 543.