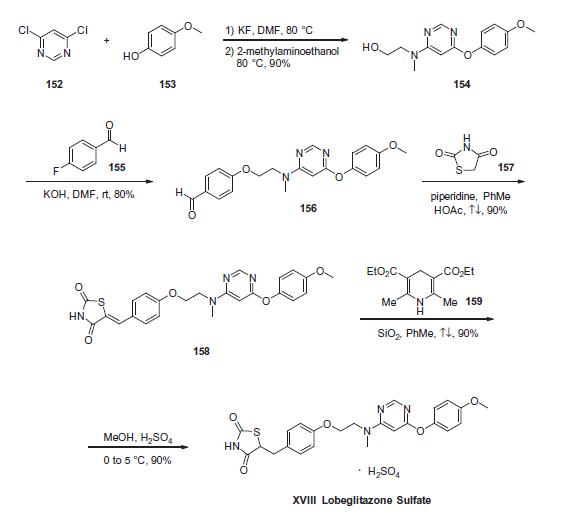
Commercially available 4,6-dichloropyrimidine (152) was treated
with a stoichiometric equivalent of p-methoxyphenol (153) in
the presence of KF in warm DMF . Upon completion of
this reaction, 2-methylaminoethanol was added to the mixture to
provide pyrimidine 154 in high yield. Next, alcohol 154 underwent
a substitution reaction with p-fluorobenzaldehyde (155)
under basic conditions to provide alkoxy benzaldehyde 156 which
was converted to the benzylidene thiazolidindione 158 upon subjection
to Knoevenagel conditions with 2,4-thiazolidinedione (157)
in 90% yield. Finally, reduction of olefin 158 was facilitated by
treatment with the Hantzsch ester (159) in the presence of silica
gel followed by treatment with methanolic sulfuric acid (96%) at
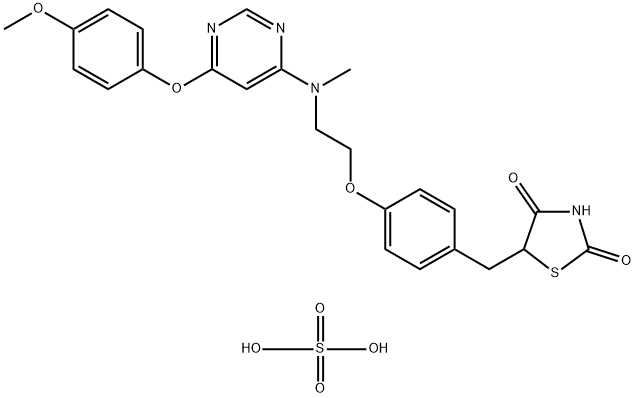
low temperature to ultimately furnish lobeglitazone sulfate in
90% yield.