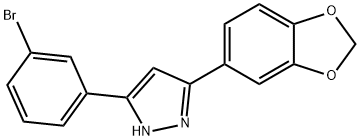
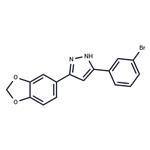
Anle138b (882697-00-9) is an aggregation inhibitor that modulates the formation of pathological oligomers of both prion and α-synuclein.1?In mouse models of prion diseases and Parkinson’s disease, Anle138b strongly inhibited oligomer formation, neuronal degeneration, and disease progression even after disease onset.1,2?Anle138b has delayed disease progression and prevented motor decline in multiple neurodegenerative models.3-6?Treatment of melanoma cells with anle138b caused massive cell death via major dysregulation of autophagy revealing a protective effect of α-synuclein on autophagy in these cells.7?Orally bioavailable and able to cross the blood-brain barrier.
Anle 138b is an anti-aggregating compound previously shown to delay the onset of scrapie, a transmissible prion disease. A protein aggregation inhibitor.
In animal studies, anle138b showed a high oral bioavailability and excellent blood-brain barrier penetration, leading to 5-fold higher levels of anle138b in the brain than in plasma. In various mouse models, anle138b strongly inhibited oligomer accumulation, neuronal degeneration, and disease progression in vivo.
Anle138b, an oligomer modulator, inhibits neuronal degeneration. It rescues neurons from the aggregation?effects of α-synuclein.
Anle138b represents a first-in-class compound that modulates toxic oligomers based on high-affinity binding to a structural epitope related to misfolding along the amyloidogenic pathway. It has been shown that compound binding destabilizes toxic oligomers, prevents the formation of oligomer pores in membranes and blocks prion-like propagation of α-synuclein aggregation. Anle138b showed structure-dependent binding to pathological aggregates and strongly inhibited the formation of pathological oligomers in vitro and in vivo for α-synuclein and other disease-relevant amyloidogenic proteins such as prion protein, Abeta, and tau. It was shown that anle138b reduces the overall number of intermolecular hydrogen bonds in oligomers, disfavors the sampling of the aggregated state, and remodels the conformational distributions within the small oligomeric peptide aggregates.
1) Wagner?et al.?(2013),?Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease;?Acta Neuropathol.,?125?795
2) Levin?et al.?(2014),?The oligomer modulator anle138b inhibits disease progression in a Parkinson’s mouse model even with treatment started after disease onset; Acta Neuropathol.,?127?779
3) Wagner?et al.?(2015),?Reducing tau aggregates with anle138b delays disease progression in a mouse model of tauopathies; Acta Neuropathol.,?130?619
4) Martinez Hernandez?et al.?(2018),?The diphenylpyrazole compound anle138b blocks Aβ channels and rescues disease phenotypes in a mouse model for amyloid pathology; EMBO Mol. Med.,?10?32
5) Heras-Garvin?et al.?(2019),?Anle138b modulates α-synuclein oligomerization and prevents motor decline and neurodegeneration in a mouse model of multiple system atrophy; Mov. Disord.,?34?255
6) Brendel?et al.?(2019),?Late-stage Anle138b treatment ameliorates tau pathology and metabolic decline in a mouse model of human Alzheimer’s disease tau; Alzheimer’s Res. Ther.,?11?67
7) Turriani?et al.?(2017),?Treatment with diphenyl-pyrazole compound anle138b reveals that α-synuclein protects melanoma cells from autophagic cell death; Proc. Natl. Acad. Sci. USA,?114?E4971