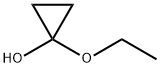
1-Ethoxycyclopropanol is used for homoenolate formation, ring expansion to β-lactams and pyrrolines via 1-aminocyclopropanols, to
cyclobutanones via 1-vinylcyclopropanols, and to cyclopentanones via 1-trimethylsiloxy-1-
vinylcyclopropanes.[1]
Preparative Methods of 1-ethoxycyclopropanol: acyloin-type reaction of ethyl 3-halopropionates with highly dispersed Sodium (or
Lithium) in refluxing Et2O in the presence of Chlorotrimethylsilane provides 1-ethoxy-1-
trimethylsiloxycyclopropane in high yields. Use of sonochemical activation simplifies the procedure.
Then, simple methanolysis (MeOH, ClSiMe3) leads to the hemiacetal (eq 1).
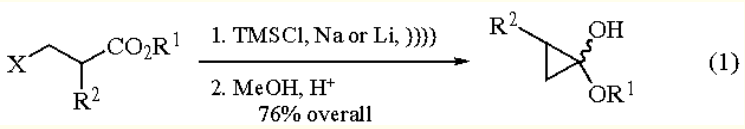
Cyclization of optically pure β-halo esters gives enantiomerically pure cyclopropanes at C-2 and a 1:1 diastereomeric mixture at C-1.
1-ethoxycyclopropanol can be kept unaltered for several months at 0 °C. On heating at 100 °C,
or on standing in acidic solvents, it undergoes ring opening to ethyl propionate.
1. (a) Salaün, J. CRV 1983, 83, 619. (b) Kuwajima, I.; Nakamura, E. Top. Curr. Chem. 1990, 155, 1. (c) Salaün, J. Top.
Curr. Chem. 1988, 144, 1. (d) Salaün, J. In The Chemistry of the Cyclopropyl Group; Rappoport, Z., Ed.; Wiley: New
York, 1987; Part 2, Chapter 13, p 809.