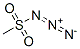Methane sulfonyl azide is used as a diazo transfer reagent for activated methylene,[1] methine,[1,2] and methyl[3] groups; functionalization of double
and triple bonds.
Preparative Methods of Methane sulfonyl azide: reaction of Methanesulfonyl Chloride with Sodium Azide in MeOH(EtOH)/H2O[1,3] or
acetone[2] (55-61 or 96% yields, respectively). By substituting acetone for methanol, the formation of
MeSO2OMe is avoided and the reagent is obtained in >95% purity.
Methane sulfonyl azide should be stored in the cold and the dark. Explosive when heated above
130 °C. No experimentalists have reported any difficulty handling MsN3, but proper caution should be used,
as with all azide reagents. Use in a fume hood.
Purification of Methane sulfonyl azide: crystallization from ether in dry-ice/acetone or from 95% EtOH at -75 °C; distillation in vacuo.
1. (a) Taber, D. F.; Ruckle, R. E.; Hennessy, M. J. JOC 1986, 51, 4077. (b) Taber, D. F.; Hennessy, M. J.; Lovey, J. P.
JOC 1992, 57, 436.
2. Danheiser, R. L.; Miller, R. F.; Brisbois, R. G.; Park, S. Z. JOC 1990, 55, 1959.
3. Shatzmiller, S.; Bercovici, S. LA 1992, 877.