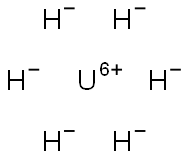Uranium hydride is a binary salt and has a molecular formula of UH3 and is a brown-gray to black powder that conducts electricity. It is highly toxic and ignites spontaneously in air.
Fine, black, pyrophoric powder. Loses H2 when heated in vacuum
above 250 °C, forming a uranium of high chemical activity. Powerful
reducing agent; reacts vigorously with water according to:
2 UH3 +
4 H2O = 2 UO2 + 7 H2.
Preparation of finely divided uranium metal
by decomposition, separation of hydrogen isotopes,
reducing agent, laboratory source of pure
hydrogen.
Uranium (10 g) is freed of the adherent oxide layer by brief
treatment with dil. nitric acid, washing with water, and drying
over P2O5. The metal is then coarsely ground and placed in a
porcelain boat, which should be large enough to accommodate the
increase in volume which accompanies the hydride formation.
Hydrogen is then passed over it at 250 °C. [The pretreatment of
the H2 includes passage through a copper column heated to 650-
700°C, a drying agent (magnesium perchlorate), and uranium
powder heated to 700-750 °C].
Highly toxic. Ignites spontaneously in air.