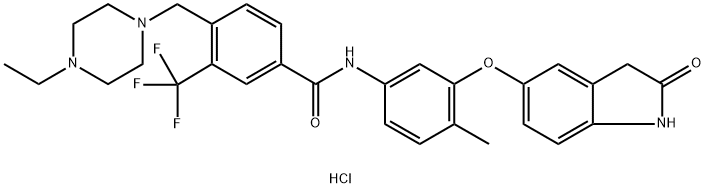
DDR1-IN-1 is a potent and selective DDR1 inhibitor with IC50 of 105 nM, about 3-fold selectivity over DDR2. DDR1-IN-1 binds to DDR1 in the 'DFG-out' conformation and inhibits DDR1 autophosphorylation in cells at submicromolar concentrations with good selectivity as assessed against a panel of 451 kinases measured. DDR1-IN-1 provides a useful pharmacological probe for DDR1-dependent signal transduction. The DDR1 receptor tyrosine kinase is activated by matrix collagens and has been implicated in numerous cellular functions such as proliferation, differentiation, adhesion, migration, and invasion.