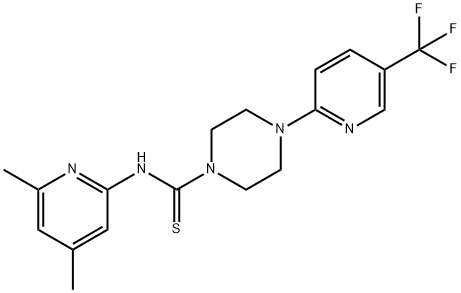
NCT-502 is a novel inhibitor of human Phosphoglycerate Dehydrogenase (PHGDH).
ChEBI: N-(4,6-dimethyl-2-pyridinyl)-4-[5-(trifluoromethyl)-2-pyridinyl]-1-piperazinecarbothioamide is a member of piperazines and a member of pyridines.
nct-502 is an inhibitor of 3-phosphoglycerate dehydrogenase (phgdh).serine, a proteinogenic amino acid, is the source of one-carbon units essential for de novo purine and deoxythymidine synthesis. homo sapiens phosphoglycerate dehydrogenase (phgdh) catalyzes the first, rate-limiting step in the canonical pathway of glucose-derived serine synthesis.
nct-502 was identified as an inhibitor of 3-phosphoglycerate dehydrogenase (phgdh), inhibiting serine synthesis from 3-phosphoglycerate in cells. nct-502 was inactive against a panel of other dehydrogenases and showed minimal cross-reactivity in a panel of 168 g-protein-coupled receptors. moreover, in mda-mb-231-phgdh cells, nct-502 treatment could decrease the intracellular serine and glycine concentrations and did not change the concentration of any other amino acid except for aspartate. in addition, nct-502-mediated inhibition of serine synthesis was found to be reversible in cells [1].
to evaluate the in-vivo activity nct-503, a structurally close analog of nct-502, nod.scid mice bearing mda-mb-231 and mda-mb-468 orthotopic xenografts were treated with vehicle or nct-503. results showed that nct-503 reduced the growth and weight of phgdh-dependent mda-mb-468 xenografts but did not affect those of phgdh-independent mda-mb-231 xenografts. phgdh inhibition caused by nct-503 also selectively increased necrosis in mda-mb-468 but not mda-mb-231 xenografts. importantly, mice treated with nct-503 did not lose weight during the 24-d treatment [1].
[1] pacold, m. e.,brimacombe, k.r.,chan, s.h., et al. a phgdh inhibitor reveals coordination of serine synthesis and one-carbon unit fate. nature chemical biology 12(6), 452-458 (2016).