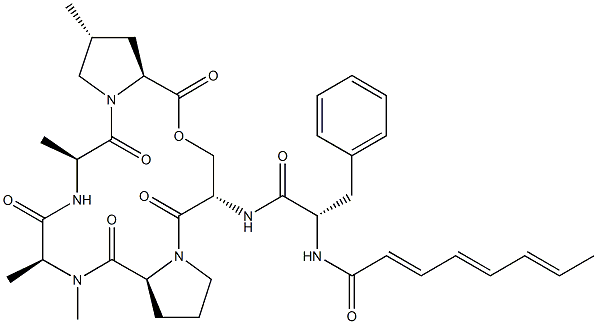
A-54556A is an acyldepsipeptide (ADEP) antibiotic that has been found in Streptomyces hawaiiensis. It is active against B. subtilis, S. pyogenes, and E. faecalis, as well as penicillin-resistant S. pneumoniae, vancomycin-resistant E. faecium, and methicillin-resistant S. aureus (MRSA; IC50s = 0.2, 0.4, 0.4, 1.6, 0.4, and 6.3 μg/ml, respectively). It binds to and activates caseinolytic protease P (ClpP), inducing degradation of β-casein by the B. subtilis ClpP in an ATPase-independent manner when used at concentrations of 2.5 and 5 μg/ml.
A 54556A is an unusual depsipeptide isolated from Streptomyces hawaiiensis by researchers at Eli Lilly in 1985, featuring a trienone side chain. A 54556A is potently active against Gram positive and Gram negative bacteria, including MRSA. A 54556A was the original lead structure of the recently re-discovered acyldepsipeptide (ADEP) antibiotics that act by activating and disregulating Clp-family proteins. ADEPs are considered important leads in the development of new generations of antibiotics against resistant bacteria.
previous study found that the treatment of b. subtilis with 1.6 mg/ml of a-54556a reduced the number of viable cells by 2 log units. in addition, the biosynthesis of dna, rna, protein, cell wall and fatty acid proceeded unhindered for 1 h at 2 mg/ml a-54556a, whereas classical antibiotics were clearly distinguished by preferential inhibition of their target pathway. microscopic examination showed that after addition of a-54556a at concentrations as low as 0.4 mg/ml, b. subtilis started to form filaments [1].
two a-54556a analogs, adep 2 and adep 4, were proven to be active in the treatment of bacterial infections in rodents. when mice were challenged with a lethal systemic infection of e. faecalis, 1 mg/kg adep 2 or 0.5 mg/kg adep 4 were sufficient for 100% survival. in lethal sepsis caused by s. aureus, 12.5 mg/kg adep 4 rescued 80% of the mice and reduced the bacterial loads in liver, spleen and lung by 2–3 log units compared to an untreated control [1].
0.2 μg/ml for bacillus subtilis 168
[1] h. brtz-oesterhelt, d. beyer, h. p. kroll, et al. dysregulation of bacterial proteolytic machinery by a new class of antibiotics. nature medicine 11(10), 1082-1087 (2005).