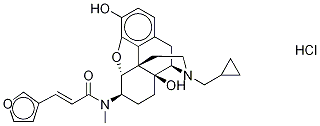
The j opioid receptor agonist nalfurafine hydrochloride was approved
and launched in 2009 for the first time in Japan. Nalfurafine
is indicated for the treatment of pruritus in hemodialysis patients
who have not responded to conventional therapies. Hemodialysis related uremic pruritus is characterized by severe systemic itching
without inflammation of the skin. Its cause has not been fully
elucidated, and it often does not respond to treatment by conventional
antipruritic drugs, such as antihistamines. Nalfurafine was
co-developed by Toray, Japan Tobacco and Torii Pharmaceuticals
and manufactured and marketed by Toray. It has orphan drug
status in Japan for the approved indication.