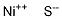Usage And Synthesis
Nickel sulfide crystalline particles occurs in nature as mineral millerite. Its principal use is as a source material for making nickel metal.
Nickel sulfide crystalline particles is mined directly from natural deposits. Also, it can be prepared in the laboratory by precipitation from an aqueous solution of a nickel salt with ammonium sulfide, (NH4)2S, or by precipitation from an acetic acid solution with hydrogen sulfide. While the aqueous solution method yields an amorphous product (alpha-NiS) which rapidly changes on exposure to air and contact with the solution to a brown crystalline sulfide (beta-NiS), the acid solution method forms only crystalline beta-NiS.
Nickel sulfide crystalline particles also can be prepared by reacting nickel powder with molten sulfur.
Nickel sulfide crystalline particles also can be prepared by reacting nickel powder with molten sulfur.
The mineral millerite is brittle; hardness, 3–3.5; specific gravity, 5.48–5.52; luster, metallic; color, brass-yellow, often with an iridescent tarnish.
Trigonal crystalline solid or amorphous powder; mineral millerite has a yellow metallic luster; color varies from yellow to brownish black; density 5.30 to 6.65 g/cm3; exhibits three allotropic modifications: (1) the acid-soluble amorphous alpha form obtained from nickel salt solution by precipitation with ammonium sulfide, (2) the alpha form rapidly transforms to a crystalline beta form as a brown colloidal dispersion upon exposure to air, and (3) a rhombohedral gamma modification found native as mineral millerite, which also can be prepared artificially under certain conditions.
Gamma-NiS slowly converts to beta-NiS in solution. Beta form probably is richer in sulfur than alpha and gamma modifications and therefore they could have varying stoichiometric compositions.
Nickel sulfide melts at 797°C and is insoluble in water (3.6 mg/L at 18°C; soluble in concentrated nitric acid and potassium hydrogen sulfide solution; slightly soluble in alcohol.
Gamma-NiS slowly converts to beta-NiS in solution. Beta form probably is richer in sulfur than alpha and gamma modifications and therefore they could have varying stoichiometric compositions.
Nickel sulfide melts at 797°C and is insoluble in water (3.6 mg/L at 18°C; soluble in concentrated nitric acid and potassium hydrogen sulfide solution; slightly soluble in alcohol.
Nickel sulfide occurs in nature as mineral millerite. Its principal use is as a source material for making nickel metal.
MILLERITE is used as an ore of nickel. Millerite was named for the English mineralogist, W.H. Miller.
Nickel sulfide is mined directly from natural deposits. Also, it can be prepared in the laboratory by precipitation from an aqueous solution of a nickel salt with ammonium sulfide, (NH4)2S, or by precipitation from an acetic acid solution with hydrogen sulfide. While the aqueous solution method yields an amorphous product (alpha-NiS) which rapidly changes on exposure to air and contact with the solution to a brown crystalline sulfide (beta-NiS), the acid solution method forms only crystalline beta-NiS.
Nickel sulfide also can be prepared by reacting nickel powder with molten sulfur.
Nickel sulfide also can be prepared by reacting nickel powder with molten sulfur.
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine