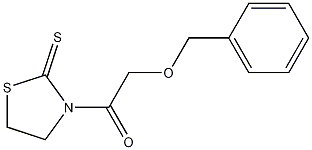
3-(2-Benzyloxyacetyl)thiazolidine-2-thione synthon for α-benzyloxy carboxylic esters, amides, or aldehydes; enolate precursor for the diastereo- and enantioselective aldol reactions.
Methyl 2-hydroxyacetate is converted to methyl 2-benzyloxyacetate (1, NaH; 2, BnBr,DMF, 0 °C to rt, 80%). The acetate is then treated with LiOH.H2O (1.25 equiv) in THF-H2O at rt to give 2- benzyloxyacetic acid (99%). The acid is converted to the corresponding acid chloride (oxalyl chloride (2.7 equiv, 60 °C, 1.5 h), which is treated with thiazolidine-2-thione in the presence of triethylamine in dichloromethane to give 3-(2-Benzyloxyacetyl)thiazolidine-2-thione (Ethanone, 2-(phenylmethoxy)-1-(2-thioxo-3-thiazolidinyl)-) (81% from the acid).