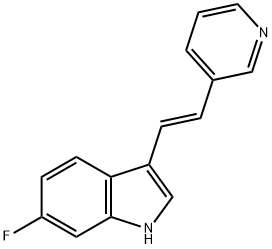
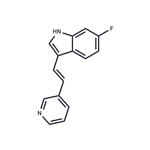
680C91 (163239-22-3) is a potent and selective inhibitor of tryptophan 2,3-dioxygenase (TDO) Ki=42 nM).1,2?Displays no activity against indoleamine 2,3-dioxygenase, MAO-A and B and does not effect serotonin uptake. Elevates CSF tryptophan by up to 260% and CSF serotonin by 170% of basal.2?TDO inhibition by 680C91 in glioma cells blocked the release of kynurenine (Cat.# 10-2666), an endogenous tumor promoting AHR ligand.4
680C91 has been used:
- as a tryptophan 2,3 dioxygenase (TDO) inhibitor to study its effects on the pigmentation in Doryteuthis pealeii embryos
- as a TDO inhibitor to study its effects on esophageal squamous cell carcinoma in xenograft tumor assay
- as a tryptophan 2,3 dioxygenase 2 (TDO2) inhibitor to study its effects on toxic fragment formation in human embryonic kidney cells
ChEBI: A fluoroindole that is 6-fluoroindole in which the hydrogen at position 3 has been replaced by a 2-(pyridin-3-yl)vinyl group (trans configuration). It is a selective inhibitor of tryptophan 2,3-dioxygenase (TDO), which directs the conversi
n of trypophan to kynurenin.
680C91 is a potent inhibitor of the enzyme tryptophan 2,3-dioxygenase (TDO), which directs the conversion of trypophan to kynurenin. Kynurenin has recently been identified as an endogenous lignd of the arylhydrocarbon receptor (AHR). TDO is highly expressed in glioma cells, and contributes to AHR-mediated glioma cell survival and suppression of anti-tumor immune responses.
1) Schwarcz and Pellicciari (2002),?Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities; J. Pharmacol. Exp. Therap.,?303?1
2) Salter?et al. (1995),?The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism; Biochem. Pharmacol,?49?1435
3) Salter?et al. (1995),?The effects of an inhibitor of tryptophan 2,3-dioxygenase and a combined inhibitor of tryptophan 2,3-dioxygenase and 5-HT reuptake in the rat; Neuropharmacology,?34?217
4) Opitz?et al. (2011),?An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor; Nature,?478197