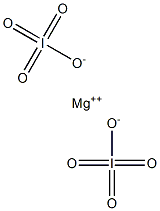
m-Magnesium periodate may be prepared by reaction of magnesium hydroxide
and periodic acid:
Mg(OH)2+2HIO4→Mg(IO4)2
The product is an octahydrate, Mg(IO4)2·8H2O. The
products differ depending upon the mode of addition.
If the acid is added to the Mg(OH)2 slurry, i.e. the
Mg2+ cation is in excess, then the meta-salt results. In the opposite in which the acid is in excess, the orthosalt, Mg(H4IO6)2, results.Magnesium periodate is not used in Industry but is
offered for sale in limited quantities commercially.