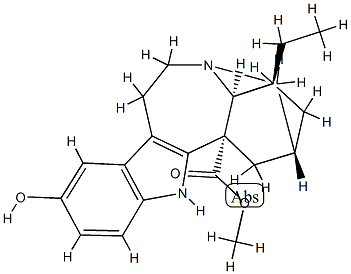
A number of hydroxylated coronaridine alkaloids have been recently isolated from certain
plants. This particular base occurs in the ethanolic extract of Tabernanthe pubescens. It is
difficult to crystallize and is laevorotatory with a specific rotation of [α] -33° (c 0.5,
CHCI3).
ChEBI: 10-hydroxycoronaridine is an organic heteropentacyclic compound that is coronaridine in which the hydrogen of the indole moiety that is para- to the indole nitrogen has been replaced by a hydroxy group. It has a role as a plant metabolite and a phytoestrogen. It is an organic heteropentacyclic compound, a monoterpenoid indole alkaloid, a methyl ester, a tertiary amino compound, a secondary amino compound and an alkaloid ester. It is functionally related to a (-)-coronaridine. It is a conjugate base of a 10-hydroxycoronaridine(1+).