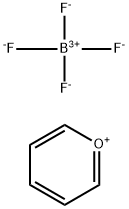
Pyrylium tetrafluoroborate is used for the functionalization of heterocyclic amines, activating them for nucleophilic substitution. As demonstrated by the Cornella lab, the intermediate pyridinium salts can react with a vast array of nucelophiles to form new C-N, C-O, and C-S bonds, making pyrylium tetrafluoroborate a valuable reagent for late-stage functionalization.