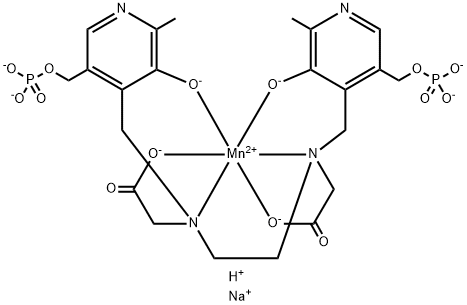
Sodium N,N'-bis(pyridoxal-5-phosphate)ethylenediimine
A 265.2 g (1 mole) of pyridoxal-5-phosphate was slurried in 1 L of methanol,
and 400 mL of 5 M NaOH was added. When the solution was homogeneous,
34.2 mL of 1,2-diaminoethane was added rapidly with vigorous stirring. The
imine product sodium N,N'-bis(pyridoxal-5-phosphate)ethylenediimine or
sodium 5-(N-(3-hydroxy-2-methyl-5-phosphonomethyl-4-pyridyl)
methylideneaminoethyleneiminomethyl)-2-hydroxy-3-methyl-5-
pyridylmethylphosphate was stirred for 1 hr, 400 mL of diethyl ether was
added, and the slurry was filtered. The filtrate was washed with 600 mL of ethanol and dried at 60°C in vacuo. A 290 g of the bis-imine with a melting
point of 215-220°C (decomp.) was isolated (90% yield, based on the tetrasodium salt).
N,N'-bis(Pyridoxal-5-phosphate)ethylenediamine
To the diimine obtained was added 1.5 L of deionized water and 1.5 L of
methanol. The yellow solution formed was stirred while sparging with
nitrogen. Then 13 g of 5% Pt on carbon was added, and the apparatus was
purged with hydrogen. The reaction was allowed to proceed for 5 hr with
continuous addition of hydrogen. HPLC analysis showed complete reduction to
the amine. The reaction mixture was sparged with nitrogen for 15 min and
then filtered through Celite. The filtrate was concentrated in vacuo at 60°C to
about 500 mL. The solution, containing N,N'-bis(pyridoxal-5-phosphate)
ethylenediamine or 5-(N-(3-hydroxy-2-methyl-5-phosphonomethyl-4-
pyridyl)methylaminoethyleneaminomethyl)-2-hydroxy-3-methyl-5-
pyridylmethylphosphoric acid salt was used directly for the next step. If
desired the diamine can be isolated as off-white crystals by the addition of
200 ml of 97% formic acid and allowing the product to crystallize at room
temperature overnight. The diamine is isolated by filtration and washed with
150 mL of cold deionized water.
N,N'-Bis-(pyridoxal-5-phosphate)ethylenediamine-N,N'-diacetic acid synthesis
The diamine obtained was dissolved in a 100 g (2.5 mole) of NaOH, and 130
g (0.9 mole) of bromoacetic acid was dissolved in 180 mL of deionized water.
Each solution was charged to an addition funnel. NaOH solution was added to
the diamine solution to bring the pH to 11. The temperature was raised to
42°C, and bromoacetic acid and NaOH solution were added concurrently to
maintain the pH at 11. The progress of the reaction was checked by HPLC. A
675 g of cation exchange resin (AMBERLITE IRC-50) was added, and the
mixture was placed in a refrigerator for 14 hr. The pH had dropped to 6.5. The
resin was removed by filtration, and the filtrate treated with 260 g of cation
exchange resin (DOWEX 50W-X8). The pH dropped to about 4. The resin was
removed by filtration, and the solution was concentrated in vacuo at 60°C to a
viscous oil. The oil was dried in vacuo for 48 hr to yield a resinous solid
containing N,N'-bis-(pyridoxal-5-phosphate)ethylenediamine-N,N'-diacetic acid
or N,N'-bis(3-hydroxy-2-methyl-5-phosphonomethyl-4-pyridylmethyl)
ethylenediamine-N,N'-diacetic acid (DPDP). The resinous solid obtained was
dissolved in 600 mL of 88% formic acid, then 1.5 L of methanol and 2.2 L of
ethanol was added, and the mixture was cooled to 0°C for 2 hr. The solvent
mixture was decanted from the resulting gum. The gum was dissolved in 800
mL of deionized water which was then concentrated in vacuo to about 600-
650 mL. Seed crystals were added, and the solution was allowed to stand
overnight. The product was isolated by filtration, washed with 400 mL of cold
water, 250 mL of ethanol, and then dried in vacuo to yield 65 g of DPDP in
85-90% purity by HPLC.
The 65 g of product was then dissolved in 75 mL of 88% formic acid
containing 5 mL of deionized water with heating to 60°C. Cold water was
added to a total volume of 1 L, and the solution was allowed to stand at 25°C
for 16 hr to crystallize. The product was isolated by filtration, washed with
200 mL cold water, and dried in vacuo at 60°C to yield 55 g of DPDP in 93-
95% purity by HPLC. A second recrystallization, using the same procedure yields 50 g of DPDP in 96-98% purity by HPLC, melting point 174-180°C
(decomp.).
Sodium salt of Mn(DPDP)
A 4.16 g (6.25 mmole) portion of DPDP was dissolved in 15 mL of rigorously
degassed water by the addition of 1.0 g (25 mmoles) of NaOH. A 1.25 g (6.25
mmole) quantity of manganese dichloride tetrahydrate was added, and the
solution immediately turned yellow. After stirring for 30 min, 0.25 g (6.25
mmole) of solid NaOH was added to bring the pH up to 6.5. Then degassed
water was added to bring the volume of the solution to 25 mL. The clear
yellow solution was sterilized by being filtered through a 0.2 micron filter to
yield the sodium salt of a manganese chelate complex of N,N'-bis-(pyridoxal5-phosphate)ethylenediamine-N,N'-diacetic acid or N,N'-bis(3-hydroxy-2-
methyl-5-phosphonomethyl-4-pyridylmethyl)ethylenediamine-N,N'-diacetic
acid.
Mangafodipirtrisodium is a clear bright to dark yellow chelate of paramagneticmanganese for intravenous administration.Manganese is distributed to the liver and pancreas and shortensthe T1-weighted relaxation time. It is indicated to detectmetastatic lesions of the liver, focal pancreatic lesions, andhepatocellular carcinoma.