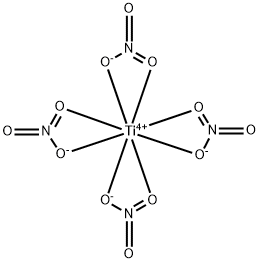
A solution of 3 ml. (5.1 g.) of TiCl4 in 10 ml. of CCl4 is cooled with Dry Ice in a two-neck flask provided with a dropping funneland a reflux condenser which is protected with a P2O5 tube. A solution of 11.6 g. of N2O5 in 25ml. of CCl4 is then added dropwise. A yellow, flocculent precipitate forms as soon as the CCl4 melts(—23°C), that is, after removal of the coolant. As the temperature rises from —23°C to room temperature, the precipitate dissolves with evolution of a gas. If too violent, the bubbling may be slowed down by cooling. Solution of the last fraction of the precipitate is accelerated by mild heating. The volatile components (NO2Cl and CCl4) are removed by vacuum distillation, leaving a residue ofthe white Ti(NO3)4, which may be purified by sublimation in high vacuum at 50°C. Part of the product decomposes in the process into N2O5 and nonvolatile TiO(NO3)2.