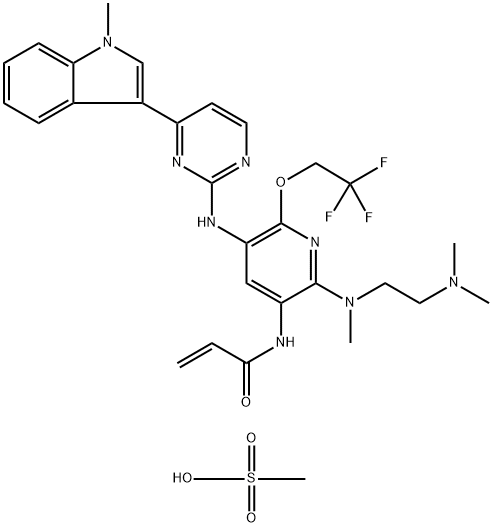
Furmonertinib (formerly known as alflutinib) is another me-too third-generation EGFR TKI that has a pyridyl ring replacing the phenyl ring of osimertinib and a trifluoroethyl group replacing the methyl group (Fig. 4). This TKI, which was discovered and developed by Shanghai Allist Pharma, was approved in November 2019 by the NMPA of China for the second-line treatment of NSCLC with EGFR sensitive mutation and EGFR T790M drug-resistant mutation, and more recently as a first-line treatment for classical EGFR mutant NSCLC. In June 2022, furmonertinib was granted the Fast Track designation by the FDA in patients with advanced or metastatic NSCLC with activating EGFR or HER2 mutations, including exon 20 insertion mutations.
Firmonertinib (Alflutinib; Furmonertinib) mesylate is is an orally active, mutant-selective, and highly brain penetrant EGFR inhibitor. Firmonertinib mesylate inhibits EGFR active mutations as well as the T790M acquired resistant mutation. Firmonertinib mesylate has the potential for the research of cancer diseases, especially advanced non-small cell lung cancer (NSCLC) with EGFR ex20ins mutation[1].
[1] Y. Shi, et al. P2.03-028 Third Generation EGFR Inhibitor AST2818 (Alflutinib) in NSCLC Patients with EGFR T790M Mutation: A phase1/2 Multi-Center Clinical Trial.
[2] Alexander I. Spira, et al. FURVENT: Phase 3 trial of furmonertinib vs chemotherapy as first-line treatment for advanced NSCLC with EGFR exon 20 insertion mutations (FURMO-004). Journal of Clinical Oncology. Volume 42, Number 16_suppl