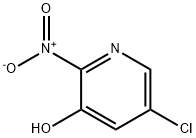
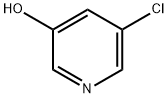
Example 15A Synthesis of 5-chloro-2-nitropyridin-3-ol: 30 g of 5-chloropyridin-3-ol (232 mmol, 1.0 eq.) was dissolved in 228 mL of concentrated sulfuric acid under ice bath cooling conditions. Keeping the temperature of the reaction system at 0 °C, 24 mL of concentrated nitric acid was slowly added dropwise. After the dropwise addition, the reaction mixture was gradually warmed to room temperature and stirred overnight. Upon completion of the reaction, the mixture was slowly poured into an ice/water mixture and stirring was continued for 30 minutes. The precipitated solid product was collected by filtration, washed with cold water and subsequently dried in air. Finally 33 g of 2-nitro-3-hydroxy-5-chloropyridine was obtained in 82% of the theoretical yield. The resulting product could be used in subsequent reactions without further purification. The product characterization data were as follows: LC-MS (Method 1): retention time Rt = 0.60 min; MS (ESneg): m/z = 172.9/174.9 ([M-H]-). 1H NMR (400 MHz, DMSO-d6): δ = 7.71 (d, 1H); 8.10 (d, 1H); 12.14 (br.s, 1H).
[1] Patent: US2014/128386, 2014, A1. Location in patent: Paragraph 1052; 1053; 1054; 1056
[2] Patent: US2014/128425, 2014, A1. Location in patent: Paragraph 0393-0397
[3] Patent: US2014/128372, 2014, A1. Location in patent: Paragraph 0414; 0415; 0416; 0417; 0418
[4] Patent: US2016/122341, 2016, A1. Location in patent: Paragraph 0545; 0546; 0547; 0548; 0549
[5] Patent: US2016/347770, 2016, A1. Location in patent: Paragraph 0522; 0523; 0524; 0525; 0526