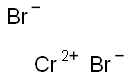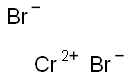(1) CrBr3 + 0.5 H2 = CrBr2 + HBr
A weighed quantity of CrBr 3 is reduced to constant weight in a
U tube at 350-400°C for 6-10 hours. The most painstaking purification of the H3 is essential for success. For uniform heating,
the U tube is surrounded with an asbestos box.
(2) Cr2(CH3COO)4 + 4 HBr = 2 CrBr2 + 4 CH3COOH
The procedure is the same as for chromium (II) chloride
(method III), but hydrogen bromide is used instead of hydrogen
chloride.