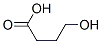
The most important physical properties of g-hydroxybutyric acid are listed in Table 2. The dissociation constant at 25℃ is 1.93×10-5. At ambient temperature, the acid is a liquid and consists in part of lactone.
Hydroxybutyric acids were utilized in the manufacture of plasticizers, but their current applications are quite limited. The related lactones are some times used as starting materials or intermediates in organic synthesis. γ-Hydroxybutyric Acid has been used as an anesthetic and pain killer. It has a stabilizing effect on blood pressure and induces a state of unconsciousness, characteristics that have encouraged its application especially in geriatrics.
ChEBI: 4-hydroxybutyric acid is a 4-hydroxy monocarboxylic acid that is butyric acid in which one of the hydrogens at position 4 is replaced by a hydroxy group. It has a role as a general anaesthetic, a GHB receptor agonist, a sedative and a neurotoxin. It is a 4-hydroxy monocarboxylic acid and a hydroxybutyric acid. It is functionally related to a butyric acid. It is a conjugate base of a 4-hydroxybutyrate.
γ-Hydroxybutyric acid is obtained from g-butyrolactone by alkaline hydrolysis and isolated as the alkali-metal salt. γ-Butyrolactone is produced by dehydrocyclization of 1,4-butanediol.