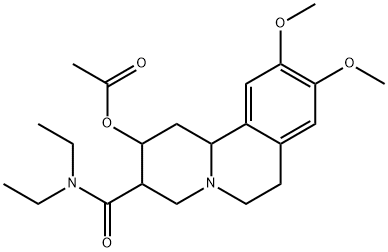
According to US Patent 3,055,894, a solution consisting of 3.4 grams
(0.01mol) of 2-oxo-3-carboethoxy-9,10-dimethoxy-1,2,3,4,6,7-hexahydro-
11b-H-benzopyridocoline and 0.8 grams (0.011 mol) of freshly distilled
diethylamine dissolved in 50 ml of xylene was refluxed under a nitrogen
atmosphere for 24 hours. After cooling to room temperature, the reaction
mixture was successively extracted with four 100 ml portions of water. The
aqueous phase was then discarded and the xylene layer was passed through a
paper filter containing a bed of sodium sulfate and activated charcoal. The
resulting filtrate was then heated under reduced pressure (65 mm Hg) via a
water bath at 50°C in order to remove the xylene solvent, and the residual oil
so obtained was cooled to approximately 5°C and held at that point until a
semisolid formed (required approximately 16 hours). Recrystallization of the semisolid from aqueous ethanol in the presence of activated charcoal afforded
light yellow crystals of 2-oxo-3-(N,N-diethylcarboxamido)-9,10-dimethoxy-
1,2,3,4,6,7-hexahydro-11b-H-benzopyridocoline, MP 150°-152°C.
Then, as described in US Patent 3,053,845, one hundred grams (0.278 mol)
of 2-oxo-3-(N,N-diethylcarboxamido)-9,10-dimethoxy-1,2,3,4,6,7-hexahydro-
11b-H-benzopyridocoline was dissolved in 1,500 ml of hot methanol and the
resulting solution was allowed to cool to room temperature. After removal of
all the dissolved oxygen therein by saturation of the solution with dry
nitrogen, 5.0 grams of Adams' platinum oxide catalyst was introduced into the
system in one portion while still maintaining same under a nitrogen
atmosphere.
The reaction flask and its contents were then shaken at room temperature
under slightly greater than one atmosphere of hydrogen pressure until the
total hydrogen uptake was completed. Dissolved hydrogen gas was then
removed from the reaction solution by saturation of same with respect to dry
nitrogen, while the platinum black was removed by means of gravity filtration.
Concentration of the resulting filtrate under reduced pressure on a steam bath
then afforded a nearly quantitative yield of 2-hydroxy-3-(N,N�diethyicarboxamido)-9,10-dimethoxy-1,2,3,4,6,7-hexahydro-11b-H�benzopyridocoline as a yellow crystalline solid (mixture of the axial and
equatorial forms).
A mixture consisting of 2 grams of 2-hydroxy-3-(N,N-diethylcarboxamido)-
9,10-dimethoxy-1,2,3,4,6,7-hexahydro-11 b-H-benzopyridocoline (OH-axial)
hydrochloride (prepared by treating the base with hydrogen chloride gas in
absolute ether) dissolved in 7 ml of acetic anhydride containing 3 ml of
pyridine was heated at 100°C for 2 hours under a nitrogen atmosphere. At
the end of this period, a crystalline precipitate had formed and the resultant
mixture was subsequently diluted with an equal volume of diethyl ether and
filtered.
The crystalline hydrochloride salt so obtained, i.e., the solid material collected
on the filter funnel, was then converted to the corresponding free base by
distribution in 10 ml of a benzene-aqueous 5% sodium carbonate system. The
product recovered from the benzene extracts was then recrystallized from
diisopropyl ether to afford 1.46 grams of 2-acetoxy-3-(N,N�diethylcarboxamido)-9,10-dimethoxy-1,2,3,4,6,7-hexahydro-11b-H�benzopyridocoline (CH3COO-axial), MP 130°-131.5°C.