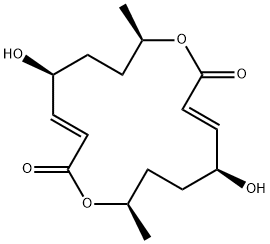
Pyrenophorol is a fungal metabolite that has been found in Alternaria and has diverse biological activities. It inhibits human topoisomerase II α when used at concentrations of 75 and 100 μM. It is active against S. cerevisiae (MIC = 4 μM) and M. violaceum. Pyrenophorol induces leaf necrosis and chlorophyll retention in wild oats when used at a concentration of 64 μM.
Pyrenophorol is a simple macrocyclic dilactone produced by a number of species of pathogenic fungi, including Byssochlamys, Stenphyllum, Alternaria and Drechslera, first reported in the late 1960s. Pyrenophorol exhibits antibiotic, herbicidal and anthelmintic properties, and is a weak inhibitor of propyl endopeptidases. Pyrenophorol inhibits seed germination but once the seed is germinated, pyrenophlorol enhances root development but causes abnormal chlorophyll retention in leaf sections.
The macrolide dilactone Pyrenophorol
n anti-fungal antibiotic produced by the plant pathogenic fungi Pyrenophora avenue and Stemphylium radicinum, which is closely related structurally to (-)-
Pyrenophorol
The macrolide dilactone Pyrenophorol was originally isolated from Byssochlamys niveah and Stemphylium radicinum. Pyrenophorol was moderately active against the fungus Microbotryum violaceum. It is an anti-fungal antibiotic produced by the plant pathogenic fungi Pyrenophora avenue and Stemphylium radicinum, which is closely related structurally to (-)-Pyrenophorol.
ChEBI: Pyrenophorol is a macrolide.