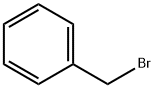
Step 3: Preparation of 4-(benzyloxy)-3-methoxy-2-nitrobenzaldehyde; 4-hydroxy-3-methoxy-2-nitrobenzaldehyde (155 g, 786 mmol) was dissolved in N,N-dimethylformamide (DMF, 1500 mL), and potassium carbonate (217 g, 1.57 mol) and benzyl bromide (161 g, 0.94 mol). The reaction mixture was stirred at room temperature for 16 h. Most of the DMF was removed by distillation under reduced pressure. the organic layer was separated by partitioning the residue between water (2 L) and ethyl acetate (EtOAc, 2 L). The organic layer was washed with saturated saline (3 x 2 L), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The resulting solid was ground with ether (Et2O, 1 L) to afford the target compound 4-(benzyloxy)-3-methoxy-2-nitrobenzaldehyde (220 g, 97% yield).1H NMR (DMSO-d6) δ: 9.77 (1H, s), 7.87 (1H, d), 7.58 (1H, d), 7.51 (1H, m), 7.49 (1H, m) , 7.39 (3H, m), 5.36 (2H, s), 3.05 (3H, s).