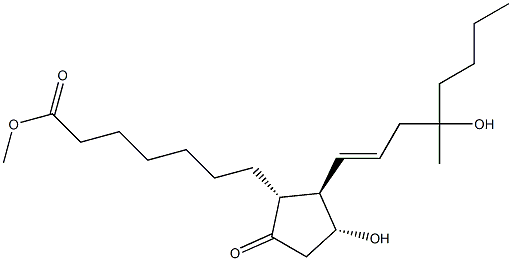
Misoprostol
- Product NameMisoprostol
- CAS62015-39-8
- MFC22H38O5
- MW382.53412
- EINECS
- MOL File62015-39-8.mol
Usage And Synthesis
ChEBI: 7-[(1R,2R,3R)-3-hydroxy-2-(4-hydroxy-4-methyloct-1-enyl)-5-oxocyclopentyl]heptanoic acid methyl ester is a prostanoid.
1 method of synthesis
To a 1000 ml dried flask under a nitrogen atmosphere was added 74.6 g of(E)-trimethyl-[[1-methyl-1-[3-(tributylstannyl)-2-propenyl]pentyl]oxy]silane,125 ml anhydrous THF and 24.2 g of copper (I) iodide. The mixture wasstirred at room temperature for 30 minutes and then it was cooled to -25 to -30°C. 98.8 ml of methyllithium (2.86 M) in DEM was added dropwise and theresultant solution was stirred at -15°C for 2 hours. Then the reaction mixturewas cooled to -78°C and 25 g of methyl-5-oxo-3-[(triethylsilyl)oxy]-1-cyclopentene-1-heptanoate in 100 ml of THF was added rapidly. After stirringthe mixture for 5 min at -78°C, it was quenched into a mixture of 750 ml ofaqueous ammonium chloride solution and 200 ml of ammonium hydroxide.The resulting mixture was warmed to room temperature and stirred until adeep blue aqueous layer was obtained. Ethyl acetate (250 ml) was used forextraction. Then the combined organic layers were washed with brine andsubsequently dried over magnesium sulfate. After a filtration andconcentration under reduced pressure, an oil (105 g) was obtained. This oilcontaining the protected prostaglandin was subjected to acidic deprotection(cat. PPTS, acetone and water) and purification (chromatography on silica gel)to provide 15.8 g (60%) of misoprostol was identical.
2 method of synthesis
To a 300 ml dried flask under a nitrogen atmosphere was added 4.45 g ofcopper (I) iodide and 60 ml of anhydrous THF. The mixture was cooled to 0°C35 ml of 1.4 M methyllithium in diethyl ether was added dropwise and theresultant solution was stirred at 0°C for 30 min. 13.7 g of (E)-trimethyl-[[1-methyl-1-[3-(tributylstannyl)-2-propenyl]pentyl]oxy]silane in 5 ml of THF wasadded and then the mixture was stirred at 0°C for 30 min. Then an additional1.5 ml of 1.4 M methyllithium in diethyl ether was added and the mixture wasstirred for another 30 min. The reaction mixture was cooled to -78°C and 10 gof methyl 5-oxo-3-[(triethylsilyl)oxy]-1-cyclopentene-1-heptanoate in 10 ml ofTHF was added rapidly. After stirring the mixture for 5 min at -78°C, it wasquenched into 210 ml of basic aqueous ammonium chloride solution. Theresulting mixture was warmed to room temperature and stirred until a deepblue aqueous layer was obtained. Ethyl acetate was used for extraction. Thenthe combined organic layers were washed with water (10 ml), then with brine(25 ml) and subsequently dried over magnesium sulfate. After a filtration andconcentration under reduced pressure, an oil (21 g) was obtained. This oilcontaining the protected prostaglandin was subjected to acidic deprotection(cat. PPTS, acetone and water) and purification (chromatography on silica gel)to provide 4.2 g (40%) misoprostol.
To a 1000 ml dried flask under a nitrogen atmosphere was added 74.6 g of(E)-trimethyl-[[1-methyl-1-[3-(tributylstannyl)-2-propenyl]pentyl]oxy]silane,125 ml anhydrous THF and 24.2 g of copper (I) iodide. The mixture wasstirred at room temperature for 30 minutes and then it was cooled to -25 to -30°C. 98.8 ml of methyllithium (2.86 M) in DEM was added dropwise and theresultant solution was stirred at -15°C for 2 hours. Then the reaction mixturewas cooled to -78°C and 25 g of methyl-5-oxo-3-[(triethylsilyl)oxy]-1-cyclopentene-1-heptanoate in 100 ml of THF was added rapidly. After stirringthe mixture for 5 min at -78°C, it was quenched into a mixture of 750 ml ofaqueous ammonium chloride solution and 200 ml of ammonium hydroxide.The resulting mixture was warmed to room temperature and stirred until adeep blue aqueous layer was obtained. Ethyl acetate (250 ml) was used forextraction. Then the combined organic layers were washed with brine andsubsequently dried over magnesium sulfate. After a filtration andconcentration under reduced pressure, an oil (105 g) was obtained. This oilcontaining the protected prostaglandin was subjected to acidic deprotection(cat. PPTS, acetone and water) and purification (chromatography on silica gel)to provide 15.8 g (60%) of misoprostol was identical.
2 method of synthesis
To a 300 ml dried flask under a nitrogen atmosphere was added 4.45 g ofcopper (I) iodide and 60 ml of anhydrous THF. The mixture was cooled to 0°C35 ml of 1.4 M methyllithium in diethyl ether was added dropwise and theresultant solution was stirred at 0°C for 30 min. 13.7 g of (E)-trimethyl-[[1-methyl-1-[3-(tributylstannyl)-2-propenyl]pentyl]oxy]silane in 5 ml of THF wasadded and then the mixture was stirred at 0°C for 30 min. Then an additional1.5 ml of 1.4 M methyllithium in diethyl ether was added and the mixture wasstirred for another 30 min. The reaction mixture was cooled to -78°C and 10 gof methyl 5-oxo-3-[(triethylsilyl)oxy]-1-cyclopentene-1-heptanoate in 10 ml ofTHF was added rapidly. After stirring the mixture for 5 min at -78°C, it wasquenched into 210 ml of basic aqueous ammonium chloride solution. Theresulting mixture was warmed to room temperature and stirred until a deepblue aqueous layer was obtained. Ethyl acetate was used for extraction. Thenthe combined organic layers were washed with water (10 ml), then with brine(25 ml) and subsequently dried over magnesium sulfate. After a filtration andconcentration under reduced pressure, an oil (21 g) was obtained. This oilcontaining the protected prostaglandin was subjected to acidic deprotection(cat. PPTS, acetone and water) and purification (chromatography on silica gel)to provide 4.2 g (40%) misoprostol.
Preparation Products And Raw materials
Raw materials
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine