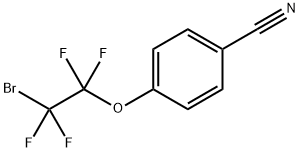
4-Cyanophenoxytetrafluorobromoethane is a fluoroalkylbromide that is a radical source of the 4-cyanophenoxytetrafluoroethyl moiety. Alternatively, it can also be selectively metallated at the fluorinated carbon with Turbo Grignard reagent at low temperatures resulting in a thermally unstable anion that can act as a nucleophilic fluoroalkylation reagent towards a wide variety of electrophiles, such as aldehydes, ketones or sulfonylimines. The nitrile group can be transformed to other derivatives, building molecular complexity.