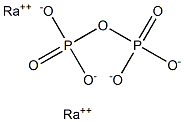
Radium pyrophosphate would have the molecular
formula of Ra2P2O7 and the molecular weight of
448.5973 g/mol. It has no CAS number. It may be
prepared by the solid-state reaction of the dibasic orthophosphate, or alternately by the precipitation of
a soluble Ba salt with aqueous pyrophosphoric acid:
2RaHPO4 + heat ? Ra2P2O7 +H2O
RaCl2 (aq) +H4P2O7 (aq) ? Ra2P2O7 (solid) + 2HCl (aq)
Radium coprecipitates with all barium compounds
(and to a lesser extent with most strontium and lead
compounds) even though the solubility product of the
radium compound itself may not be exceeded. Because
radium was one of the first radioactive elements utilized
in tracer research, it was used in the development of the
coprecipitation laws. Thus, in view of the intense radioactivity
of 226Ra, the formation of (Ba,Ra)2P2O7 is probably
the best method of forming the pyrophosphate.
The only usage of radium pyrophosphate has been in
the medical field as a radioactive tracer and catalyst.